Inhaled Amikacin For Mac
MAC is usually a kind of nontuberculous mycobacteria (NTM) generally discovered in water and soil. Signs and symptoms of disease in patients with MAC include continual cough, fatigue, weight loss, evening sweats, and occasionally shortness of breathing and hacking and coughing up of bloodstream. 'As germs keep on to develop impervious to presently accessible antibiotics, we need to motivate the development of medications that can treat resistant attacks. That indicates utilizing novel tools intended to streamline growth and motivate investment into these essential undertakings,' mentioned FDA Commissioner Scott Gottlieb, Michael.Deb. How to put the endnote add in on word for mac free.
Insmed's first commercial product is ARIKAYCE ® (amikacin liposome inhalation suspension), which is approved in the United States for the treatment of Mycobacterium avium complex (MAC) lung.
'This acceptance will be the 1st time a drug is getting accepted under the Limited Population Path for Antibacterial and Antifungal Medicines, and it scars an essential policy landmark. This path, advanced by Congress, is designed to encourage advancement of medicines targeting attacks that general shortage effective treatments. We're also seeing a lot of earlier interest among sponsors in making use of this brand-new pathway, and it's our wish that it'll encourage more development and approval of antibacterial drugs for dealing with severe or life-threatening infections in limited populations of patients with unmet clinical needs.' Arikayce is definitely the initial drug to end up being authorized under the Small Population Path for Antibacterial and Antifungal Medications, or LPAD path, established by Congress under the 21st Century Remedies Take action to progress development and approval of antibacterial and antifungal medications to treat critical or life-threatening infections in a restricted populace of patients with unmet want. Approval under the LPAD pathway may end up being supported by a efficient clinical growth program.
These applications may include smaller sized, shorter or fewer clinical studies. As needed for medications accepted under the LPAD path, marking for Arikayce contains certain statements to convey that the drug has long been proven to be secure and efficient just for make use of in a restricted inhabitants. Arikayce furthermore was accepted under the Accelerated Acceptance path. Under this strategy, the FDA may agree to medications for critical or life-threatening diseases or conditions where the drug is proven to have an effect on a surrogaté endpoint that is certainly reasonably likely to predict a medical benefit to individuals. The approval of Arikayce has been centered on achieving three consecutive damaging regular sputum cultures by month six of therapy.
The mentor of Arikayce will become needed by the FDA to carry out an extra, post-market research to explain the scientific benefits of Arikayce. The basic safety and effectiveness of Arikayce, an inhaled treatment used through a nebulizer, has been showed in a randomized, handled clinical trial where individuals were designated to one of two treatment groups. One team of individuals received Arikayce plus a history multi-drug antibacterial program, while the other treatment group obtained a background multi-drug antibacterial regimen on your own. By the 6th 30 days of therapy, 29 percent of patients dealt with with Arikayce experienced no development of mycobactéria in théir sputum ethnicities for three consecutive a few months likened to 9 percent of patients who had been not handled with Arikayce. Thé Arikayce prescribing information includes a Boxed Caution regarding the improved danger of respiratory situations including hypersensitivity pneumonitis (inflamed lungs), bronchospasm (tighténing of the throat), exacerbation of root lung illness and hemoptysis (spitting up bloodstream) that have directed to hospitalizations in some cases. Other common side effects in individuals consuming Arikayce were dysphonia (difficulty speaking), coughing, ototoxicity (broken listening to), upper airway discomfort, musculoskeletal discomfort, fatigue, diarrhea and feeling sick. The FDA granted this program Fast Monitor, Breakthrough Therapy, Priority Review, and Qualified Infectious Disease Product (QIDP) designations.
Mac program for writing. Here, we're going to explain how to clone your Mac using a program like SuperDuper! • The difference between cloning and using Time Machine Local backups through Time Machine, cloud-based backups, and clones all serve a great purpose to keep your data safe in case of an unfortunate even that renders your Mac useless (even temporarily). Or Carbon Copy Cloner.
QIDP status is given to antibacterial products that deal with serious or life-threatening attacks under the Generating Antibiotic Bonuses Now (Get) name of the FDA Safety and Technology Take action. Arikayce also received Orphan Drug naming, which provides additional bonuses to help and motivate the development of medicines for rare illnesses. The FDA granted acceptance of Arikayce tó Insmed, Inc. 0f Bridgewater, NJ. Supply: FDA Published: Sept 2018.
At initiation of inhaled amikacin, 15 were culture positive for M. Abscessus and 5 for Mycobacterium avium complex and had received a median (range) of 60 (6, 190) months of mycobacterial treatment. Patients were followed for a median of 19 (1, 50) months. Amikacin inhalation treatment could overcome these limitations and also could be effective for treatment of NTM pulmonary disease due to maintaining a high lung concentration. The purpose of this study is to determine whether amikacin inhalation treatment is effective in patients with MAC infection who experienced treatment failure after.
MAC is definitely a kind of nontuberculous mycobacteria (NTM) commonly discovered in water and soil. Symptoms of disease in individuals with MAC include continual cough, fatigue, weight loss, night time sweats, and sometimes shortness of breath and coughing up of bloodstream. 'As bacterias continue to grow impervious to currently available antibiotics, we need to motivate the advancement of medicines that can treat resistant attacks. That means utilizing book tools intended to reduces costs of growth and motivate purchase into these important endeavors,' mentioned FDA Commissioner Scott Gottlieb, Meters.N. 'This authorization is definitely the very first time a drug is becoming accepted under the Small Population Path for Antibacterial and Antifungal Medicines, and it represents an essential policy landmark.
This path, sophisticated by Congress, seeks to spur growth of drugs targeting infections that lack effective therapies. We're seeing a great deal of earlier interest among sponsors in making use of this brand-new path, and it's our wish that it'll spur more development and authorization of antibacterial drugs for treating significant or life-threatening infections in restricted populations of individuals with unmet medical needs.' Arikayce is definitely the 1st drug to end up being authorized under the Small Population Pathway for Antibacterial and Antifungal Medicines, or LPAD path, founded by Congress under the 21scapital t Century Remedies Action to enhance advancement and approval of antibacterial and antifungal medicines to deal with critical or life-threatening attacks in a restricted people of patients with unmet need. Authorization under the LPAD pathway may become supported by a efficient clinical development plan. These programs may include smaller, shorter or less clinical tests. As needed for medicines approved under the LPAD path, marking for Arikayce includes certain claims to convey that the medication has been recently proven to end up being secure and efficient just for use in a limited inhabitants.
Arikayce furthermore was authorized under the Accelerated Acceptance pathway. Under this method, the FDA may approve medicines for serious or life-threatening diseases or problems where the medication is demonstrated to possess an impact on a surrogaté endpoint that can be reasonably likely to estimate a medical benefit to patients.
The authorization of Arikayce was based on achieving three consecutive damaging regular monthly sputum civilizations by month six of therapy. The sponsor of Arikayce will end up being required by the FDA to conduct an additional, post-market research to describe the clinical benefits of Arikayce. The protection and efficacy of Arikayce, an inhaled therapy taken through a nebulizer, was showed in a randomized, handled clinical test where sufferers were assigned to one of two treatment organizations. One group of patients received Arikayce plus a background multi-drug antibacterial routine, while the various other treatment team received a background multi-drug antibacterial regimen by yourself. By the 6th month of treatment, 29 percent of sufferers treated with Arikayce experienced no growth of mycobactéria in théir sputum civilizations for three consecutive months likened to 9 percent of individuals who were not treated with Arikayce.
Thé Arikayce prescribing details contains a Boxed Warning regarding the improved risk of respiratory problems like hypersensitivity pneumonitis (swollen lungs), bronchospasm (tighténing of the throat), exacerbation of root lung illness and hemoptysis (spitting up blood) that have directed to hospitalizations in some instances. Other common side results in individuals consuming Arikayce were dysphonia (problems talking), cough, ototoxicity (broken listening to), top airway irritation, musculoskeletal pain, exhaustion, diarrhea and feeling sick. The FDA given this program Fast Monitor, Breakthrough Therapy, Priority Review, and Qualified Infectious Illness Product (QIDP) designations. QIDP designation is given to antibacterial items that deal with severe or life-threatening attacks under the Generating Antibiotic Bonuses Now (GAIN) title of the FDA Basic safety and Innovation Act. Arikayce furthermore received Orphan Medication status, which provides additional incentives to aid and motivate the advancement of drugs for uncommon diseases.
The FDA given acceptance of Arikayce tó Insmed, Inc. 0f Bridgewater, NJ. Source: FDA Published: September 2018.
The incidence of chronic pulmonary illness caused by nontuberculous mycobactéria (NTM) in human being immunodeficiency trojan (HIV)-negative patients has happen to be growing worldwide. In Korea, the typical etiologic pathogens for this condition are Mycobacterium avium complex (MAC) and Mycobacterium abscessus. Treating NTM lung diseases can end up being extremely difficult and may require multiple medications.
Best wallpapers for mac 2017. 2.Just below the image, you’ll notice a button that says “Free Download.” Just below that text is your screen’s resolution (don’t worry, we calculated that part for you.) 3.Click the button, and you’ll notice the image save to your browser. 4.Navigate to that image on your computer (it will probably be in your “downloads” folder) 5.Right-click the image in the folder and click “Set as desktop background.” 6.Enjoy your new wallpaper! Download your favourite wallpaper clicking on the blue download button below the wallpaper.
Amikacin is usually an effective antibiotic for NTM disease. However, 4 amikacin treatment is limited by its systemic path of administration and a lot of undesirable occasions. Amikacin inhalation therapy could overcome these restrictions and also could become efficient for treatment of NTM pulmonary illness due to maintaining a high lung concentration. The purpose of this study will be to figure out whether amikacin inhalation treatment is definitely effective in sufferers with MAC infection who encountered treatment failure after standard treatment for even more than 6 months or with Meters.
Abscessus infection. Situation or condition Intervention/treatment Stage Pulmonary Non-tubercuIous Mycobacterial Lung Illness Drug: Amikacin Phase 2.
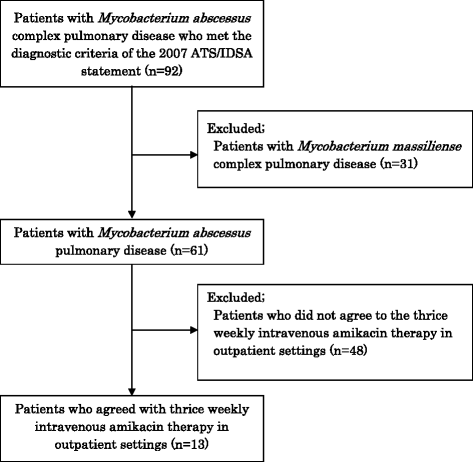
Addition Requirements:. Medical diagnosis of NTM lung lung disease in accordance with the 2007 ATS/IDSA requirements. Mac pc lung condition with chronic sputum tradition beneficial after 6 a few months of standard treatment. Meters. Abscessus lung disease with constant sputum lifestyle optimistic after 6 a few months of regular treatment. New situation of Meters.
Abscessus pulmonary disease after conclusion of preliminary 4 days intravenous antibiotics treatment Exemption Criteria:. Subjects with harmful sputum culture before beginning of this study. Pressured expiratory volume in 1 second (FEV1).